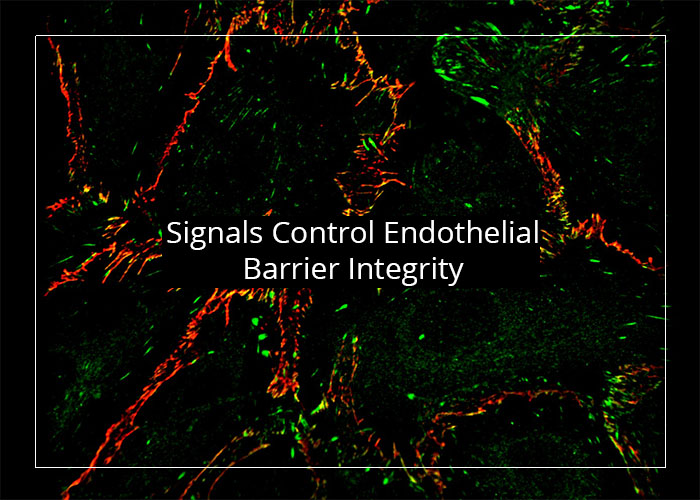
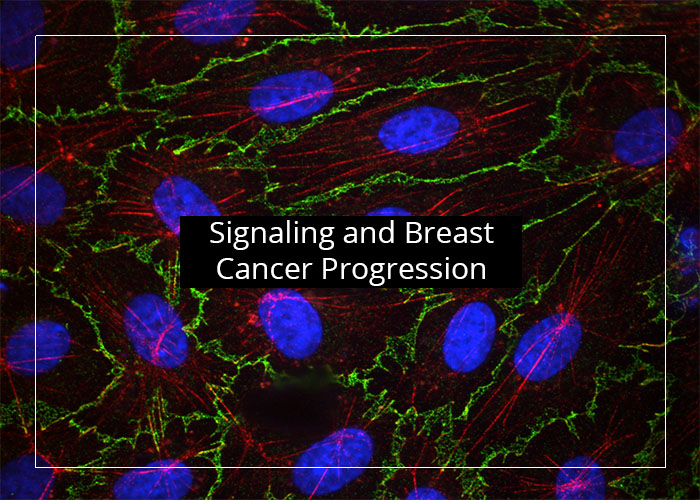
Our research is focused on cell signaling and how it regulates vascular inflammation, endothelial barrier leakage and breast cancer progression. We employ cell and molecular biology, biochemical and microscopy-based approaches using cell and animal model systems.
The TREJOlab recognizes and appreciates the support from the following sponsors
![]()




